- The value of Trust
- Midni Respect and deliver the value of trust and Contribution to everyone's clean and green life.
Product
Water Treatment System
- Home
- Product
- >
- Water Treatment System
- >
- RO/UF System
RO / UF SYSTEM
Reverse Osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. IF normal osmosis takes place, the fresh water will cross the membrane to dilute the concentration solution. in reverse osmosis, pressure is exerted on the side with the concentrated solution to forece the water molecules across the membrane to the fresh water side. The pore size of the membrane is less than 1/10,000 micrometer and can remove suspended solids, particulates, salts, bacteria, total organic carbons.
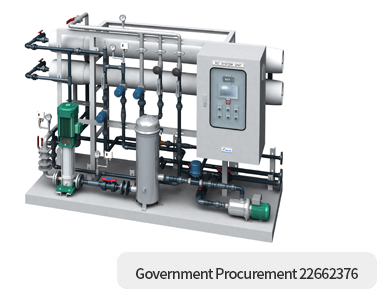
- RO System
- Raw Water Feed Pump
- Micro Filter
- RO High Pressure Pump
- RO Vessel, Membrane
- Conductivity Meter, PH Meter
- Flow Indicators
- Flushing, control Valves
- Control Panet(PLC, Touch Screen)

- UF System
- Raw Water Feed Pump
- Bag Filter
- UF Membrane
- CIP System
- Chemical Dosing System
- Control Panel(PLC, Touch Screen)

